We are ready to help you overcome the challenges of producing and developing innovative biotechnological products and advancing scientific research
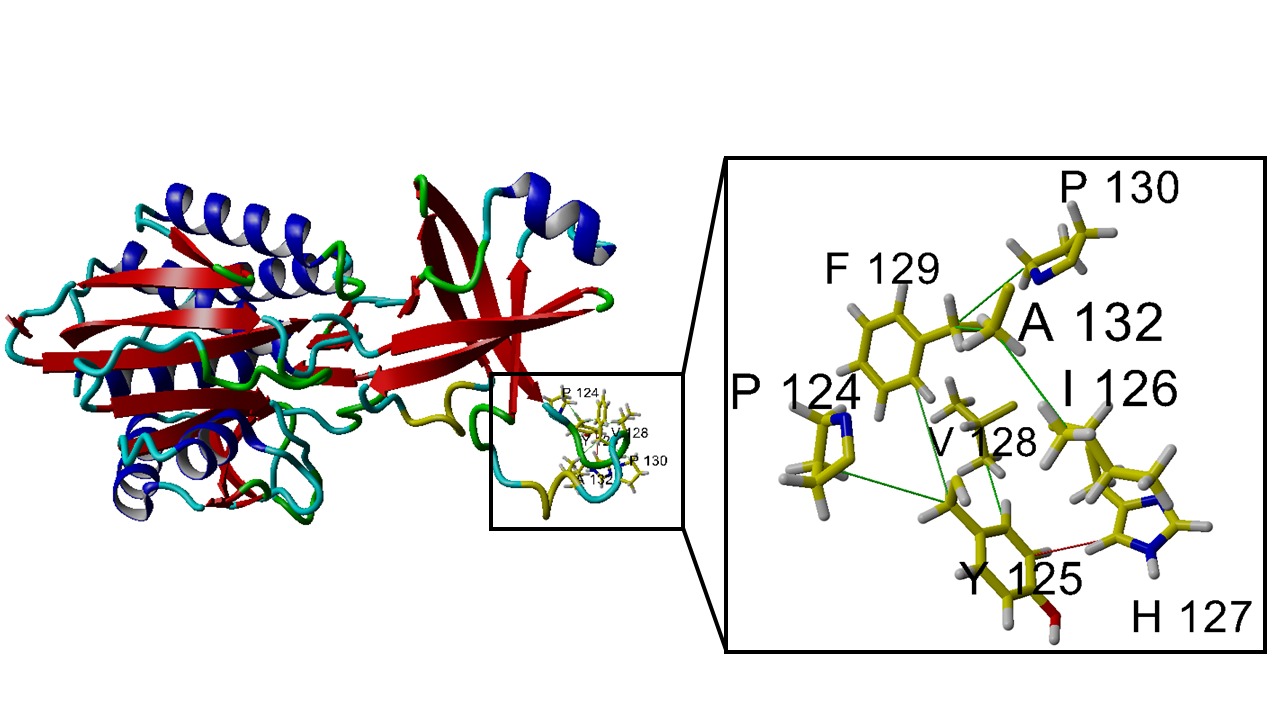
Loops are flexible regions located between the protein’s secondary structure elements, such as alpha-helices and beta-sheets, and they are often found on the protein’s surface. While the flexibility of these regions can be beneficial for certain dynamic functions, excessive loop movement frequently leads to reduced overall protein stability, increased aggregation, or even decreased enzyme activity
Reducing loop flexibility by designing mutations that introduce hydrogen bonds, ionic bonds, or hydrophobic interactions can stabilize the structure of these regions, consequently improving the enzyme’s thermal stability and overall function. This strategy is widely used, particularly in enzymes employed under harsh industrial conditions

One of our specialized services in targeted protein engineering is the design and formation of disulfide bonds between cysteine residues within the protein’s three-dimensional structure. Leveraging advanced bioinformatics tools, our team identifies optimal positions for disulfide bond formation and uses rational mutation design to enhance the protein’s thermal and structural stability. This service is particularly effective and efficient for enzymes used under harsh industrial conditions (such as high temperatures or pH levels). Our goal is to provide solutions that significantly improve protein stability without compromising catalytic performance.

At Smart Protein Builders, one of our specialized services in intelligent protein design is B-Factor Saturation Mutagenesis. In this method, we utilize protein structural information (such as PDB files) to identify regions with a high B-factor, which indicates increased mobility or flexibility.
We then target these regions using saturation mutagenesis (systematic replacement with all or a selected group of amino acids) to enhance enzyme stability or activity.
This strategy allows for the identification and optimization of residues that have the greatest impact on protein performance, using the actual 3D structure as the basis for decision-making. By combining this method with rigorous screening assays and predictive energy analyses, our team delivers effective and targeted solutions for improving protein performance and stability.

At our company, one of the advanced services in targeted protein engineering is the design of mutations aimed at increasing the volume of the enzyme’s catalytic pocket. This method is particularly relevant for enzymes that must react with larger, modified, or non-natural substrates.
By enlarging the internal space of the catalytic pocket through the replacement of bulky amino acids with smaller or more flexible ones, we facilitate better substrate access to the active site and reduce steric hindrance.
Our team utilizes 3D structural modeling, molecular dynamics analysis, and active pocket volume calculation to design mutations that enhance the reaction rate and increase the enzyme’s catalytic activity while preserving the structural integrity. This strategy plays a key role in developing industrial enzymes capable of acting on a broader range of substrates.

At our company, one of the advanced protein engineering strategies is Charge Change Optimization, which is performed with the aim of improving the stability, solubility, and functional activity of proteins.
This process involves identifying and modifying charged amino acids on the surface or in the internal regions of the protein. The goal is to optimize charge distribution to reduce unfavorable electrostatic repulsions or to enhance beneficial interactions.
By utilizing computational analyses such as electrostatic potential calculation and molecular dynamics modeling, we design targeted mutations to ensure the protein’s structure and function are maintained under industrial conditions like high temperature or pH. This service is highly effective, especially for enzymes used in specialized or industrial environments
We are ready to partner with you in overcoming production challenges, developing innovative biotechnological products, and advancing scientific research


At our company, one of the specialized strategies for improving enzyme performance is the introduction of targeted mutations in regions near the catalytic residues, specifically by replacing a highly hydrophilic amino acid with a hydrophobic residue.
This approach achieves two things: it increases the structural stability of the enzyme by strengthening the hydrophobic core around the active site, and it simultaneously enhances the enzyme’s affinity for binding hydrophobic ligands.
The design of such mutations is based on detailed analysis of the 3D structure, examination of the active environment’s polarity distribution, and molecular dynamics simulation. In many cases, adding a hydrophobic residue at an appropriate position leads to significant improvements in hydrophobic substrate binding, increased reaction yield, and enhanced enzyme stability under harsh conditions. This service is one of our key pathways for developing enzymes with optimal industrial performance.

One of our specialized services in protein engineering is the designing of mutations that lead to the creation or strengthening of stable hydrogen bonding networks within the enzyme’s three-dimensional structure. These bonds play a crucial role in maintaining spatial structure, reducing unwanted flexibility, and increasing the protein’s resistance to harsh environmental conditions such as temperature, pH, or organic solvents. Utilizing advanced bioinformatics tools and structural analysis, our team identifies regions susceptible to hydrogen bond formation and designs the appropriate amino acids for mutation. These are positioned at key locations to establish new bonds with side or main chains. This strategy is highly effective and applicable, particularly in optimizing industrial and therapeutic enzymes that require high stability under stressful conditions.
+98 912 836 0916
+98 930 144 1004
nimanezhad86@gmail.com


Custom-optimized rational enzyme engineering for your process requirements
©۲۰۲5 .neoenzyme all rights reserved